We are interested in deciphering the molecular mechanisms of cell invasion shared between the trophoblast and cancer cells.
The placenta is a transient organ that connects the embryo to the mother during pregnancy and mediates nutrient and oxygen supply to sustain normal growth. Hence, the placenta orchestrates developmental outcomes and fetal growth. Functionality of the placenta depends on the earliest steps during placentation when trophoblast cells, the building block of the placenta, invade into the endometrium to establish the definitive maternal-fetal interface. Several pregnancy complications such as miscarriage and preeclampsia are caused by defects in the process of trophoblast invasion.
Intriguingly, trophoblast cells share some key similarities with carcinomas. These similarities include the ability to invade healthy tissues, the formation of new vessels and the promotion of an immunotolerant environment. A key biological question remains: do tumour cells repurpose the same genes and mechanisms that are critical for trophoblast invasion?
By using CRISPR/Cas9 genome editing systems, we will genetically manipulate trophoblast stem cells and organoids to:
- Identify the molecular signatures characteristics of invasive placental cells.
- Unravel the common molecular pathways between trophoblast invasion and cancer metastasis.
Our results will provide fundamental insights into the cellular invasive mechanisms that coordinate placentation and the potential implication of these same mechanisms in tumour metastasis.
Presentation
Get to know us better
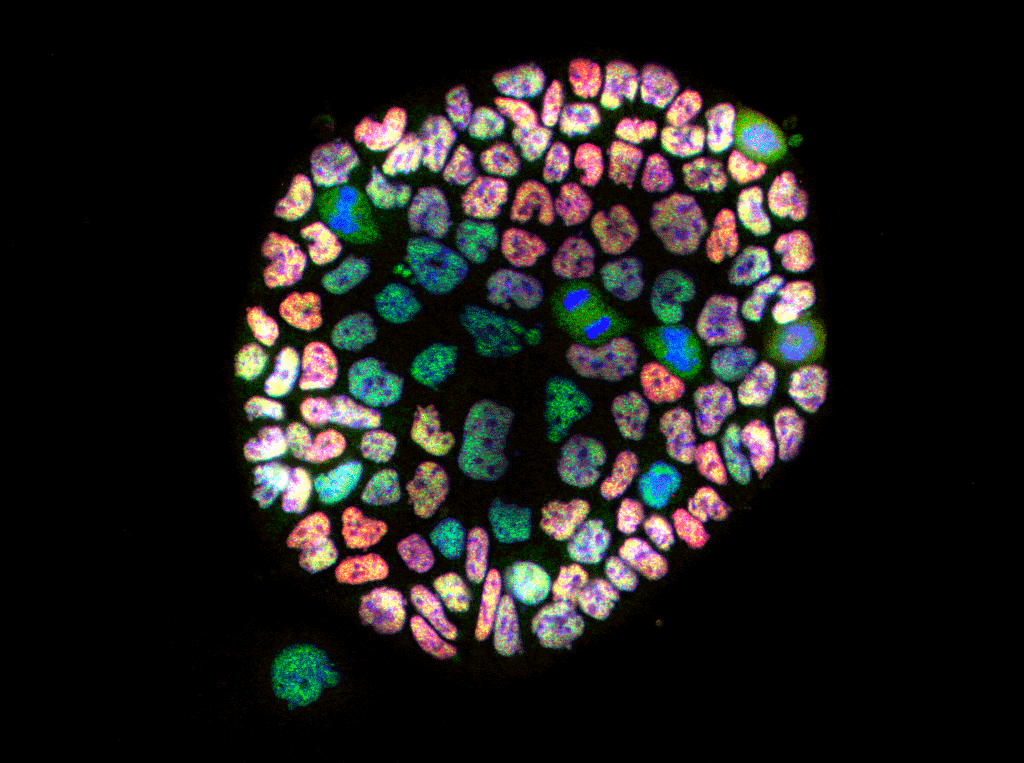
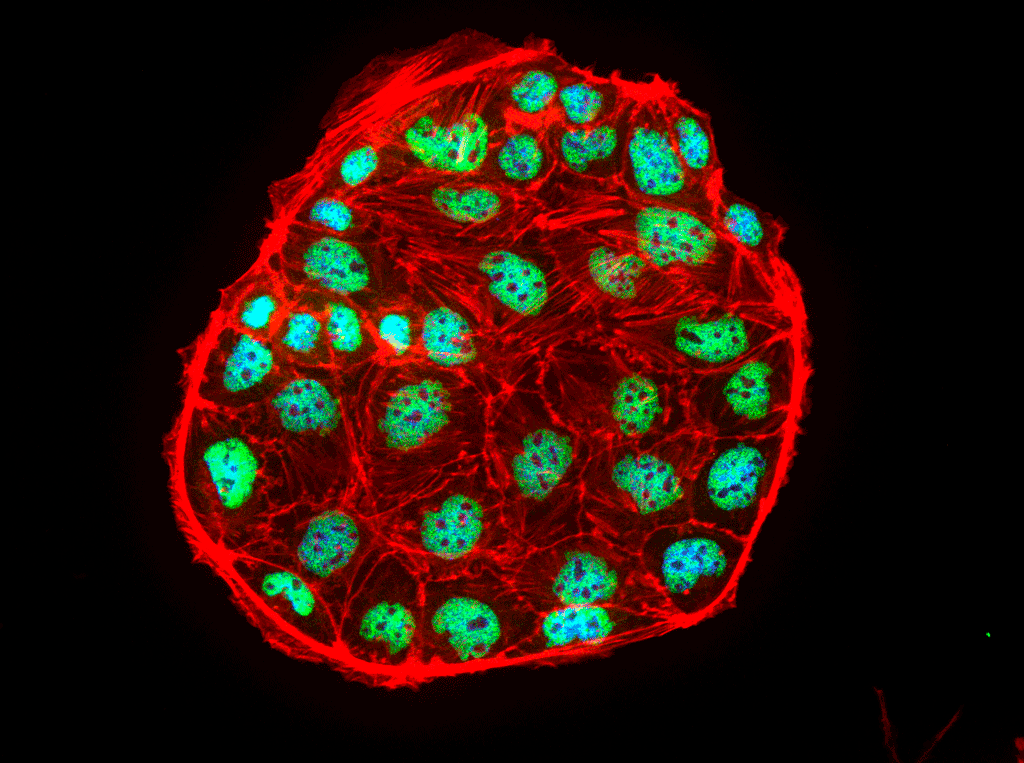
Research Staff
The people who make it all possible
Vicente Pérez García
vperez@cipf.es
Sergio Navarro Serna
snavarro@cipf.es
Maravillas Mellado López
mmellado@cipf.es
Paula Doria Borrell
pdoria@cipf.es
Ana Ferrero Mico
aferrero@cipf.es
Publications
Our scientific contributions
BAP1/ASXL complex modulation regulates epithelial-mesenchymal transition during trophoblast differentiation and invasion.
Perez-Garcia V, Lea G, Lopez-Jimenez P, Okkenhaug H, Burton GJ, Moffett A, Turco MY and Hemberger M
eLife, 2021 Jun, DOI: 10.7554/eLife.63254, Vol. 10, pag.
Placentation defects are highly prevalent in embryonic lethal mouse mutants.
Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, Sienerth A, White JK, Tuck E, Ryder EJ, Gleeson D, Siragher E, Wardle-Jones H, Staudt N, Wali N, Collins J, Geyer S, Busch-Nentwich EM, Galli A, Smith JC, Robertson E, Adams DJ, Weninger WJ, Mohun T and Hemberger M
NATURE, 2018 Mar, DOI: 10.1038/nature26002, Vol. 555, pag. 463-468
Decidualisation and placentation defects are a major cause of age-related reproductive decline.
Woods L, Perez-Garcia V, Kieckbusch J, Wang X, DeMayo F, Colucci F and Hemberger M
Nature Communications, 2017 Sep, DOI: 10.1038/s41467-017-00308-x, Vol. 8, pag. 352-352
Keep Calm and the Placenta Will Carry On.
Perez-Garcia V and Turco MY
DEVELOPMENTAL CELL, 2020 Aug, DOI: 10.1016/j.devcel.2020.06.031, Vol. 54, pag. 295-296
Regulation of Placental Development and Its Impact on Fetal Growth-New Insights From Mouse Models.
Woods L, Perez-Garcia V and Hemberger M
Frontiers in Endocrinology, 2018 Sep, DOI: 10.3389/fendo.2018.00570, Vol. 9, pag. 570-570
FUNDING
Thank you for supporting us