We use Drosophila melanogaster to study the basic biological mechanisms underlying development and disease.
The group is part of the UPV-CIPF Joint Unit, established in 2016 to promote scientific collaborations between researchers in both institutions in the fields of pathophysiology and nanomedicine. We use Drosophila melanogaster to study the basic biological mechanisms underlying development and disease.
We are using Drosophila to generate models to study rare diseases, with an especial interest in inherited peripheral neuropathies and in Dravet syndrome and othe rare epileptic encephalopathies.. Our ultimate goals are to understand the disease mechanisms involved and to generate new tools for biomarker and drug discovery. To achieve these goals, we have a network of collaborators that include groups working in Drosophila genetics, physiology and rare diseases; and we are also starting collaborations with clinical groups and patient associations.
We are using genome editing techniques with the aim to develop new disease mechanisms that can be used in the discovery of new biomarkers and treatments for rare diseases. The goal is to replace the Drosophila gene with the equivalent human gene carrying clinical mutations found in patients. This way we can develop strategies in personalized and precision medicine.
Presentation
Get to know us better
Research Staff
The people who make it all possible
Máximo Ibo Galindo Orozco
igalindo@cipf.es
Maria Del Carmen Martin Carrascosa
mcmartin@cipf.es
Christian Palacios Martínez
cpalacios@cipf.es
Publications
Our scientific contributions
Oxidative Stress, a Crossroad Between Rare Diseases and Neurodegeneration.
Espinós C, Galindo MI, García-Gimeno MA, Ibáñez-Cabellos JS, Martínez-Rubio D, Millán JM, Rodrigo R, Sanz P, Seco-Cervera M, Sevilla T, Tapia A and Pallardó FV
Antioxidants, 2020 Apr, DOI: 10.3390/antiox9040313, Vol. 9, pag.
The Drosophila junctophilin gene is functionally equivalent to its four mammalian counterparts and is a modifier of a Huntingtin poly-Q expansion and the Notch pathway
E. CALPENA, V. DEL AMO, M. CHAKRABORTY, B. LLAMUSI, R. ARTERO, C. ESPINOS and M. GALINDO
Disease Models & Mechanisms, 2018 Jan, DOI: 10.1242/dmm.029082, Vol. 11, pag.
A Drosophila model of GDAP1 function reveals the involvement of insulin signalling in the mitochondria-dependent neuromuscular degeneration
V. DEL AMO, M. PALOMINO-SCHATZLEIN, M. SECO-CERVERA, J. GARCIA-GIMENEZ, F. PALLARDO, A. PINEDA-LUCENA and M. GALINDO
BIOCHIMICA ET BIOPHYSICA ACTA-MOLECULAR BASIS OF DISEASE, 2017 Mar, DOI: 10.1016/j.bbadis.2017.01.003, Vol. 1863, pag. 801-809
Evolutionary History of the Smyd Gene Family in Metazoans: A Framework to Identify the Orthologs of Human Smyd Genes in Drosophila and Other Animal Species
E. CALPENA, F. PALAU, Carmen Espinós and M. GALINDO
Plos One, 2015 Jul, DOI: 10.1371/journal.pone.0134106, Vol. 10, pag.
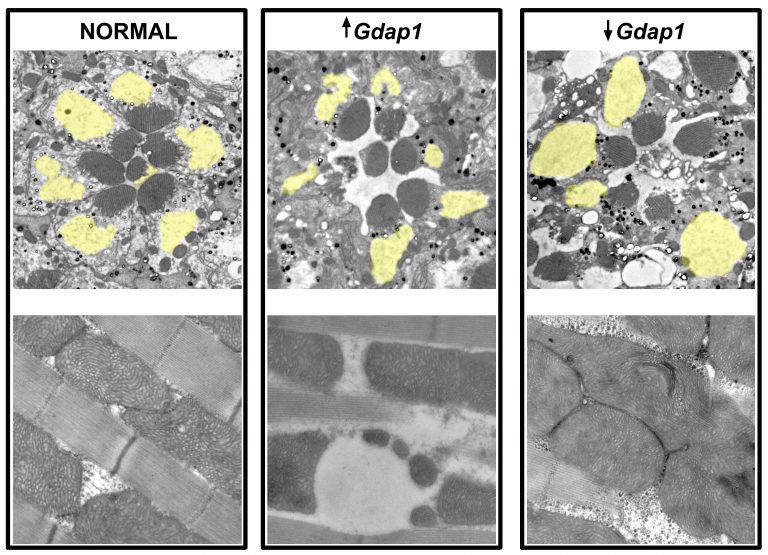
Mitochondrial defects and neuromuscular degeneration caused by altered expression of Drosophila Gdap1: implications for the Charcot-Marie-Tooth neuropathy
V. DEL AMO, M. SECO-CERVERA, J. GARCIA-GIMENEZ, A. WHITWORTH, F. PALLARDO and M. GALINDO
HUMAN MOLECULAR GENETICS, 2015 Jan, DOI: 10.1093/hmg/ddu416, Vol. 24, pag. 21-36
FUNDING
Thank you for supporting us